What We Do
Our work is inspired by the rich history of heavy elements, the mysteries surrounding non-traditional stable isotope fractionation, and the fascinating world of planktonic organisms.
Mercury and sulfur have captivated scientists and philosophers alike since the Middle Ages. The early studies of mercury-sulfur mixtures by alchemists can even be interpreted as the earliest descriptions of a chemical bond. Over 2000 years later, we remain fascinated by the chemistry of these elements. In my group, we also delve into the chemistry of actinides and lanthanides.
Current Projects
Theoretical Chemistry
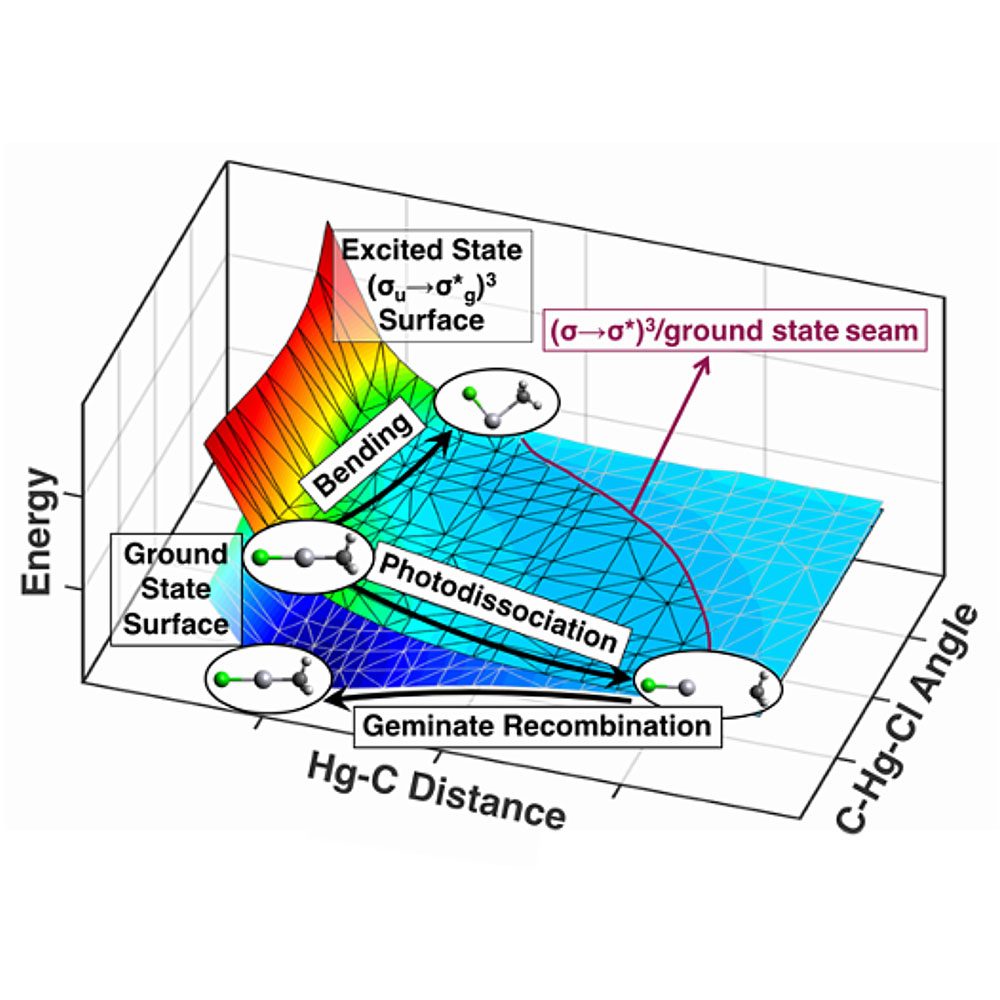
MIF remains one of the most intriguing phenomena in isotope biogeochemistry, with the largest signals observed in photochemical reactions. Despite their critical role in applications ranging from oceanography to volcanology, no tractable theory currently exists to explain the mechanisms underlying these effects. To address this challenge, our group develops new quantum chemical methodologies for predicting photochemical MIF. As lead PI on an NSF Chemical Theory grant, we are advancing theory and non-adiabatic molecular dynamics to explain and predict large MIF signatures. Our goal is to construct the first theoretical model that incorporates isotope properties such as nuclear spin to unlock the origin of elusive MIF in S, Hg, O, and N.

Synthesis and Photochemistry
Experimental evidence for the role of isotopic properties beyond mass—nuclear volume, hyperfine coupling/nuclear spin, and quadrupole moments—in MIF remains limited. This gap has hindered our ability to understand the mechanisms behind key Earth and environmental processes that yield MIF. Hg and S stable isotopes offer a particularly compelling test case for advancing our understanding of MIF. Both undergo active photolysis in nature, are biogeochemically active, and result in large MIF. Because Hg and S exhibit strong affinity and are often bonded together in nature, MIE theory suggests that if Hg undergoes MIF during photolysis, the same mechanism should also alter S. This makes Hg-S a platform for probing the isotope chemistry of two enigmatic elements.
Analytical Chemistry

Theoretical or laboratory experiments are not perfect representations of our complex planet, and it is critical to determine whether the proposed mechanisms are active in nature. To that end, we use isotopic fingerprints to evaluate previously unknown or poorly understood Earth processes. Our fieldwork specializes in Hg stable isotope biogeochemistry to investigate the underexplored compartments of the cycle of this global pollutant. We are one of few labs in the United States capable of making precise Hg stable isotope measurements, a capability that has led to several firsts, including mercury isotope analyses of marine zooplankton and particles, as well as uncovering the formation pathways of methylmercury in the ocean, and its connections to atmospheric chemistry and the transfer of anthropogenic Hg emissions to marine biota—ultimately informing public health concerns.
