U/Ca Partitioning Between Aragonite and Seawater
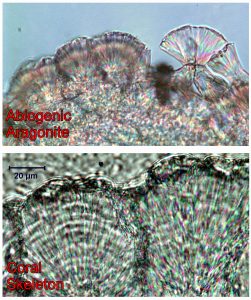
The aragonite skeleton of scleractinian corals contain coherent seasonal and interannual variability in U/Ca. In field-sampled corals and those grown in controlled culture experiments, strong correlations have been found between coral skeleton U/Ca and water temperature, pH, carbonate ion concentration, and salinity. However, the mechanism(s) underlying these different correlations remain unclear. We performed abiogenic precipitation experiments designed to evaluate the sensitivity of U partitioning between aragonite and seawater to temperature, pH, and the concentration of carbonate ion in seawater. Aragonite was precipitated from seawater by addition of carbonate alkalinity at rates set to maintain stable carbonate chemistry during precipitation. Experiments were conducted at 20 to 40 °C, with pH 7.8–9.0 and carbonate ion concentrations of 600–2600 mmol kg-1. U/Ca of the bulk precipitate and fluid were determined by inductively coupled plasma mass spectrometry. Our results show that the U/Ca ratio of aragonite precipitated from seawater decreases with increasing carbonate ion concentration, and is independent of pH and temperature. We use these results as a framework to interpret the skeletal composition of coral aragonite precipitated from a calcifying fluid that is semi-isolated from the external seawater environment. Accordingly, coral U/Ca ratios are consistent with calcifying fluid carbonate ion concentrations that are several times greater than those of ambient seawater. Correlations between coral U/Ca ratios and seawater temperature, carbonate chemistry, and other environmental variables arise indirectly, via the impacts of these variables on the carbonate ion concentration of the coral calcifying fluid.
Funding for this research is provided by The National Science Foundation (NSF OCE-1338320).