Upper Mantle Oxygen Fugacity
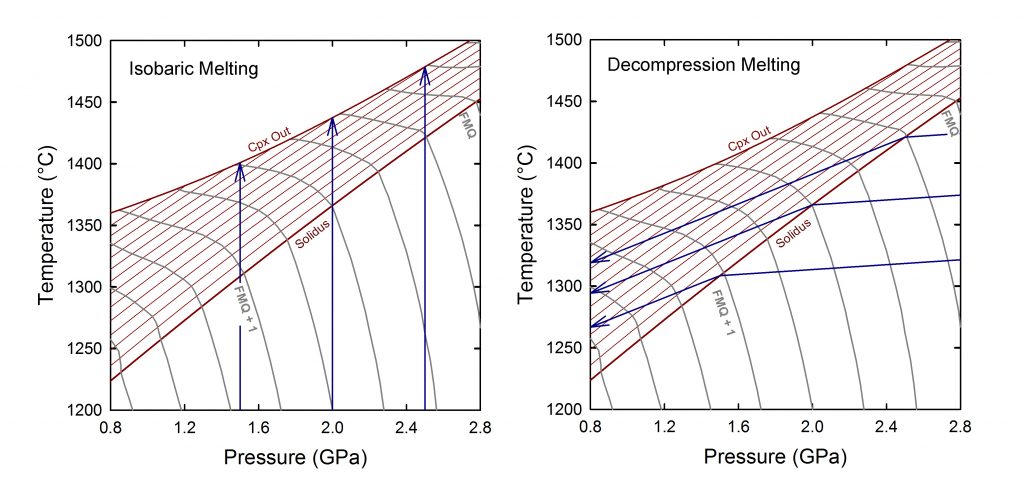
The fugacity of oxygen in the oceanic upper mantle is a measure of the amount of oxygen available to react with elements – such as iron and carbon – that can exist in multiple valence states. Knowledge of upper mantle oxygen fugacity is important because it controls a number of parameters that significantly impact our interpretation of geophysical data and our understanding of mantle dynamics. Iron is the highest concentration redox-sensitive element in basalts and peridotite, existing in both relatively oxidized (trivalent iron or Fe3+) and reduced (divalent iron or Fe2+) forms. The ratio of trivalent-to-total iron (Fe3+/SFe) can be determined in basaltic glass using X-ray absorption near edge structure (XANES) spectroscopy, and then related to oxygen fugacity using equilibrium thermodynamics. This makes the Fe3+/SFe ratio of basaltic glass the most commonly used proxy for oxygen fugacity of the asthenospheric upper mantle. I developed a model to describe the behavior of Fe3+/SFe during partial melting of the oceanic upper mantle. Results from this model provide a framework within which the Fe3+/SFe ratios of basalt glasses can be related to the oxygen fugacity regime of the oceanic upper mantle. Results from isobaric batch melting calculations demonstrate that the Fe3+/SFe ratio of the partial melt decreases with increasing melt fraction. Conversely, the Fe3+/SFe ratio of the partial melt increases with increasing melt fraction during decompression batch melting. The relative oxygen fugacity of the upper mantle depends on both the oxidation state of iron and mantle potential temperature. Results from incremental decompression melting calculations in which 1% melt is produced for each 100 MPa of decompression and then removed from the residue indicate that relative oxygen fugacity calculated from the oxidation state of iron in basaltic glass does not represent a unique value for the oceanic upper mantle but, rather, reflects conditions in the lower portion of the melting regime. A 100 °C change in mantle potential temperature produces a change in relative oxygen fugacity of ~0.8 log units, similar to the global range inferred from mid-ocean ridge basalt glasses. It is necessary, therefore, to compare relative oxygen fugacity calculated from basaltic glass with proxies for potential temperature before drawing conclusions on heterogeneity of the oxidation state of iron in the oceanic upper mantle.
Funding for this research is provided by an Independent Research and Development grant from the Woods Hole Oceanographic Institution and The National Science Foundation (NSF OCE-1459649).