What we do
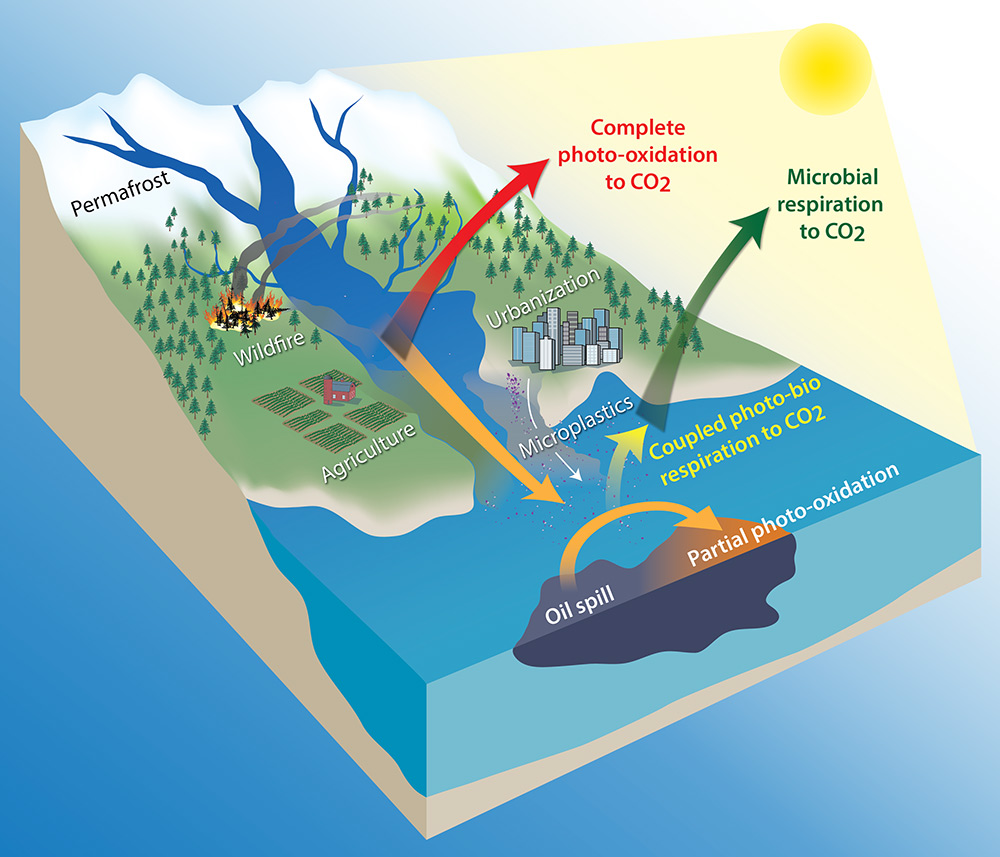
The Ward Lab largely studies four processes that transform organic carbon in aquatic ecosystems. First, microbes use organic carbon as a food source and respire it to carbon dioxide (CO2; green arrow). Second, sunlight converts organic carbon to CO2 in a process known as photomineralization or complete photochemical oxidation (red arrow). Third, sunlight transforms organic carbon into new, oxygenated compounds in a process known as partial photochemical oxidation (orange arrow). Lastly, some of these partially photo-oxidized compounds are more labile to microbes and can be respired to CO2 in a process known as coupled photo-bio degradation (yellow arrow). We study these processes for a wide range of organic carbon types, including naturally derived organic carbon, like dissolved organic matter, and organic pollutants, like crude oil and plastics.
Current projects
Crude Oil
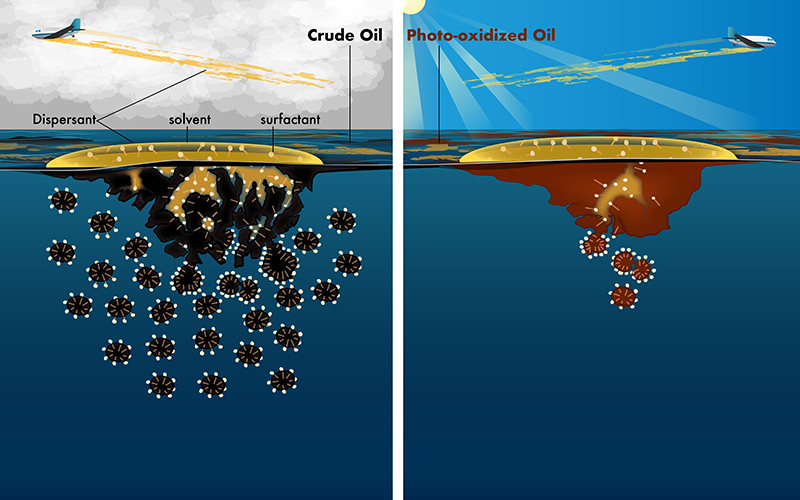
Sunlight changes the physical and chemical properties of oil spilled into the ocean. We discovered that approximately half of the oil floating on the Gulf of Mexico after the 2010 Deepwater Horizon disaster was partially oxidized by sunlight in less than one week. This oxidation has important implications for the longer-term fate of the oil and how to effectively respond to oil spills. We are currently focused on understanding the controls of photochemical reactions, which will enable a more predictive understanding of the photochemical fate of oil spilled into surface waters.
Plastics
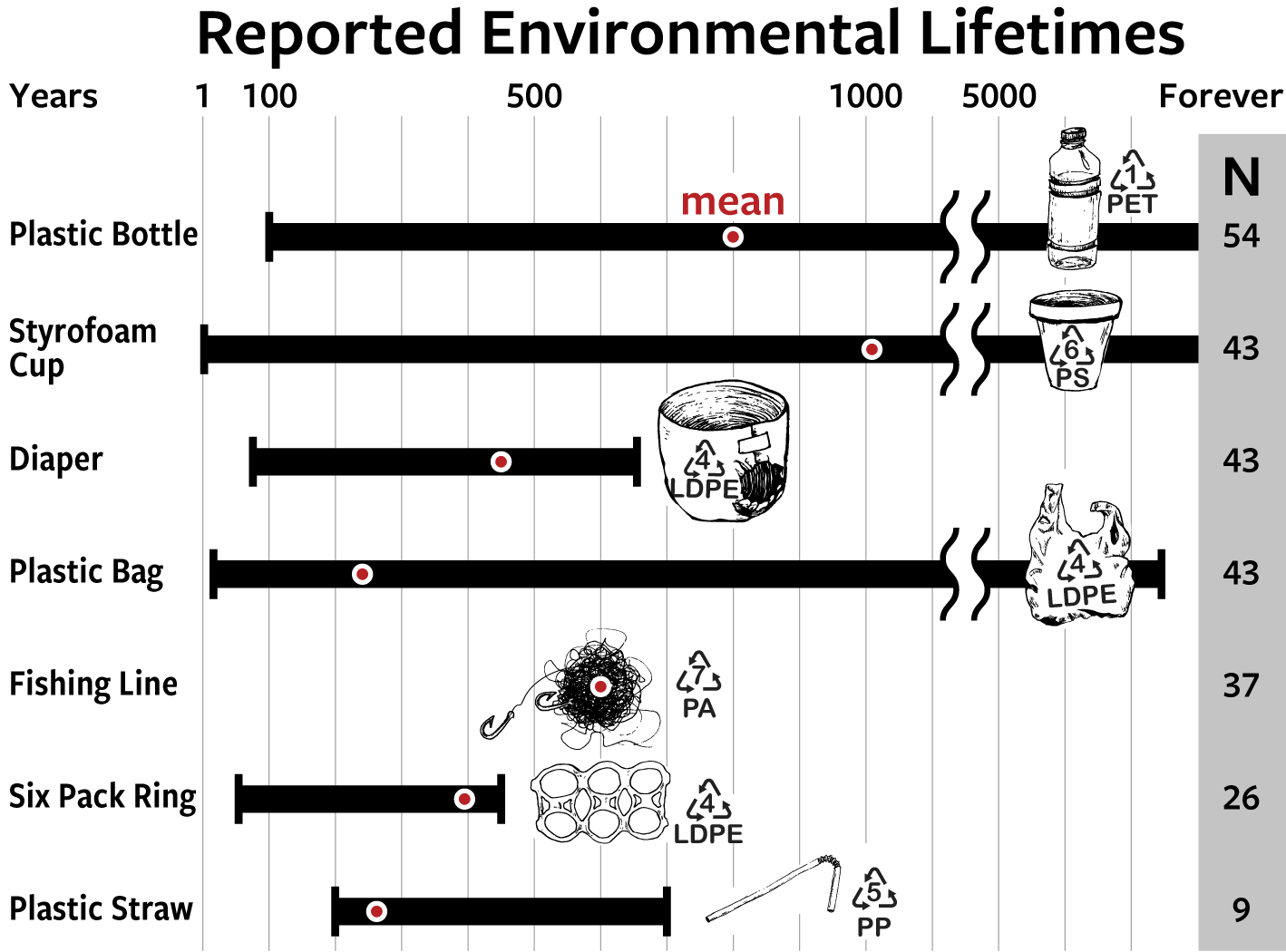
Our goal is to better understanding the timescales, products, and controls of plastic degradation in the environment, information that can be leveraged into next-generation materials that are useful and also degrade when leaked into the environment. We have multiple projects that collectively work towards achieving this goal. Two projects funded by The Seaver Institute and NSF Environmental Chemical Sciences focus on identifying what controls the fate of common consumer products in the sunlit surface ocean. Another project, funded by Eastman Chemical, aims to identify functional, sustainably sourced plastic materials that demonstrably degrade in the environment.
Instrument Development
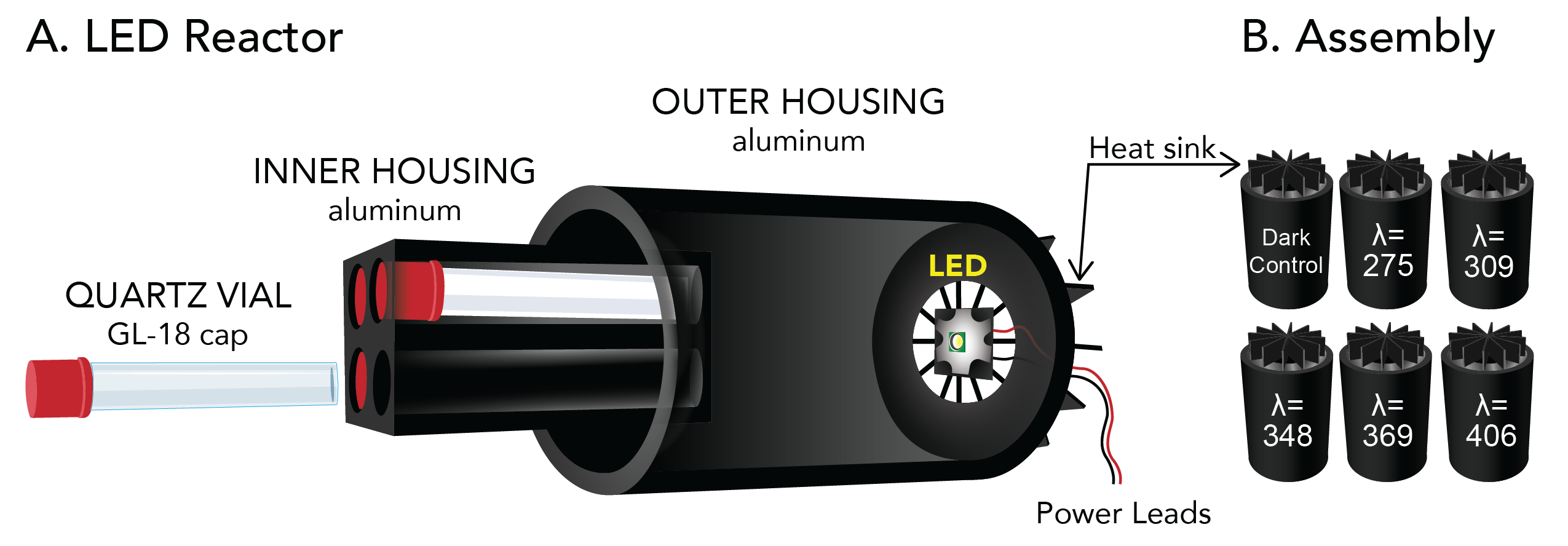
We are actively exploring new ways to study the degradation of organic carbon in surface waters. Technologies we are developing include (i) LED based methods to probe the wavelength dependence of photochemical reactions, (ii) a prototype incubator to measures in-situ rates of photochemical oxidation, microbial respiration, and primary production, and (iii) novel approaches to isolate dissolved organic matter from surface waters.
Dissolved Organic Matter
Dissolved organic matter (DOM) is a key intermediate in the global carbon cycle. Sunlight alters the chemical composition and fate of DOM in surface waters. We study the rates, pathways, and controls of these light-driven reactions, with focus on photochemical oxidation (O2 consumption) and mineralization (CO2 production).