Project 3: Cellular and molecular mechanisms underlying long-term effects of early life exposure to HAB toxins
Project Lead: Dr. Mark E. Hahn
Project Co-Investigators: Drs. John Stegeman, Neel Aluru, Sibel Karchner, and Jennifer Panlilio.
Students: Ms. Alia Hidayat, Ms. Jordan Pitt
Research Assistant: Ms. Chesna Mandl
Collaborators: Dr. Ed Levin (Duke University); Dr. Wendy Heiger-Bernays (Boston University School of Public Health)
The overall objective of the research in this project is to elucidate the cellular and molecular mechanisms by which early-life exposure to harmful algal bloom (HAB) toxins interferes with neurodevelopment to cause persistent neurobehavioral changes later in life. The HAB toxins domoic acid and saxitoxin occur in seafood and levels are regulated to prevent acute toxicity. However, human exposure to these toxins at levels below regulatory limits is common, widespread, and may be increasing, posing risks to vulnerable subpopulations such as developing humans. It is now well known that the early life environment can profoundly influence health throughout the life course (the developmental origins of health and disease). However, the mechanisms by which developmental exposures elicit effects later in life are not well understood. To elucidate these mechanisms, we are using zebrafish, a powerful model system in developmental neurotoxicology research.
Domoic acid (DA)
We used the zebrafish embryolarval model system to investigate how early life exposure to low doses of DA affects the developing nervous system [1]. Zebrafish embryos exposed to DA at 2 days post-fertilization (dpf), but not at 1 or 4 dpf, showed deficits in startle response behavior at 7 dpf (Fig. 1). Similarly, myelination in the spinal cord was disorganized after exposure at 2 dpf but not at 1 or 4 dpf (Fig. 2). Time-lapse imaging revealed disruption of the initial stages of axon myelination. Whole-embryo RNA-sequencing showed that DA exposure at 2 dpf down-regulated genes required for maintaining myelin structure and the axonal cytoskeleton. The results identified a developmental window of susceptibility to DA-induced neurodevelopmental effects and suggested that the mechanisms involve altered gene expression and disrupted myelination.
Subsequently, we used the well-known startle response circuit as a tool to identify specific neuronal components that are targeted by early life exposure to DA [2]. Exposure of 2-dpf embryos to DA reduced their responsiveness as larvae to both auditory/vibrational and electrical stimuli; there was a dramatic reduction in short-latency c-starts (SLCs). We found that sensory inputs to the startle circuit were intact in DA-exposed larvae, but that the reticulospinal neurons responsible for SLCs (Mauthner cells) were absent in most of the exposed larvae (Fig. 3). The results raised the question of whether neurons or myelinating oligodendrocytes are the initial targets of DA.
Recently [3], we sought to address this question by examining the earliest cellular changes caused by embryonic DA exposure. Here, we found that although exposure of zebrafish embryos to DA at 2 dpf did not reduce the number of oligodendrocyte precursor cells prior to myelination, it later led to fewer myelinating oligodendrocytes that produced shorter myelin sheaths and appeared to aberrantly wrap neuron cell bodies (Fig. 4). However, larvae exposed to DA lacked Mauthner neurons even prior to the onset of myelination, suggesting that the loss of the axons is not secondary to myelin defects. Experiments involving co-exposure to DA and GANT61, which reduces oligodendrocyte number, showed that the inappropriate myelination of neuronal cell bodies was likely caused by the loss of the axonal targets. These results suggest that DA alters reticulospinal neurons first and the loss of axons causes aberrant myelination of nearby cell bodies. The identification of initial targets and perturbed cellular processes provides a mechanistic understanding of how DomA alters neurodevelopment, leading to structural and behavioral phenotypes.
Additional research explored the effect of exposure to DA on gene expression in brain of adult zebrafish. Zebrafish were exposed to DA by intracelomic injection at a dose that caused transient neurological symptoms (“symptomatic dose”) or a dose that did not cause such effects (“asymptomatic dose”) and then sampled at 24, 48 and 168 h post-injection. Transcriptional profiling revealed distinct, non-overlapping changes in gene expression between the two doses. Surprisingly, asymptomatic exposure produced more persistent transcriptional effects. The results suggest that transcriptional responses are specific to the DA dose and that asymptomatic exposure can cause long-term changes [4].
Saxitoxin (STX)
We performed experiments to investigate the developmental effects of exposure to saxitoxin. Microinjection of STX (24 or 48 pg) or vehicle (0.3 mM HCl) at 6 hpf did not cause any overt toxicity but observed transient loss of pigmentation between ~36-48 hpf and it was resolved by 72 hpf. Bulk-RNAseq was conducted on 24, 36 and 48 hpf to determine transcriptional changes post-exposure. No differentially expressed genes (DEGs) were observed at 24hpf but thousands of DEGs were observed at 36 and 48hpf. KEGG pathway analysis revealed significant enrichment of genes related to focal adhesion, adherens junction and regulation of actin cytoskeleton, suggesting that cell-celland cell and extracellular matrix (ECM) interactions were affected by STX exposure. Using transgenic zebrafish, we confirmed the impact of altered ECM genes on motor neuron branching. Confocal imaging of STX-exposed 96hpf larvae suggests significant effect on caudal primary motor neuron branching. However, we did not observe any significant effect on startle response behavior in the exposed larvae. Further studies are needed to understand the impact of exposure on ECMs.
Mechanisms of developmental neurotoxicity of PCBs
In addition to examining the impacts of HAB toxins on developmental processes, we also used zebrafish to explore mechanisms involved in effects of a group of persistent marine toxicants–polychlorinated biphenyls (PCBs) [5-12]. These studies address the impact of exposure to dioxin and non-dioxin like PCBs during development and pre-conceptional window of development as well as latent effects of early life exposure to PCBs. Together, these studies carried out as part of Project 3 have advanced our understanding of the mechanisms by which marine toxins and toxicants can disrupt neurodevelopment, providing a foundation for the research proposed here.
Exposure modeling
Ongoing research in collaboration with Projects 1 and 2, the Community Engagement Core, and Dr. Wendy Heiger-Bernays (Boston University) is modeling human exposure to domoic acid and saxitoxin and how it may change with a changing climate (Fig. 5).
Overall, this research will identify the cellular and molecular bases for neurobehavioral effects following early-life exposure to prominent HAB toxins, contributing to an understanding of the potential long-term health consequences of developmental exposure to domoic acid and saxitoxin in humans, critical for assessing public health risks associated with the possibly increasing exposure to these toxins.
References cited:
- Panlilio JM, Aluru N, Hahn ME (2020) Developmental Neurotoxicity of the Harmful Algal Bloom Toxin Domoic Acid: Cellular and Molecular Mechanisms Underlying Altered Behavior in the Zebrafish Model. Environ Health Perspect 128: 117002. https://ehp.niehs.nih.gov/doi/10.1289/EHP6652
- Panlilio JM, Jones IT, Salanga MC, Aluru N, Hahn ME (2021) Developmental Exposure to Domoic Acid Disrupts Startle Response Behavior and Circuitry in Zebrafish. Toxicol Sci 182: 310-326. https://academic.oup.com/toxsci/article/182/2/310/6294322
- Panlilio JM, Hammar KM, Aluru N, Hahn ME (2023) Developmental exposure to domoic acid targets reticulospinal neurons and leads to aberrant myelination in the spinal cord. Scientific reports. 13(1):2587. https://doi.org/10.1038/s41598-023-28166-2
- Hidayat AS, Lefebvre KA, MacDonald J, Bammler T, Aluru N (2022) Symptomatic and asymptomatic domoic acid exposure in zebrafish (Danio rerio) revealed distinct non-overlapping gene expression patterns in the brain. Aquat Toxicol 252: 106310.
- Aluru N, Engelhardt J (2022) Exposure to 3,3',4,4',5-Pentachlorobiphenyl (PCB126) Causes Widespread DNA Hypomethylation in Adult Zebrafish Testis. Toxicol Sci 188: 75-87.
- Aluru N, Karchner SI, Krick KS, Zhu W, Liu J (2018) Role of DNA methylation in altered gene expression patterns in adult zebrafish (Danio rerio) exposed to 3, 3', 4, 4', 5-pentachlorobiphenyl (PCB 126). Environ Epigenet 4: dvy005.
- Aluru N, Krick KS, McDonald AM, Karchner SI (2020) Developmental Exposure to PCB153 (2,2',4,4',5,5'-Hexachlorobiphenyl) Alters Circadian Rhythms and the Expression of Clock and Metabolic Genes. Toxicol Sci 173: 41-52.
- Glazer L, Hahn ME, Aluru N (2016) Delayed effects of developmental exposure to low levels of the aryl hydrocarbon receptor agonist 3,3',4,4',5-pentachlorobiphenyl (PCB126) on adult zebrafish behavior. Neurotoxicology 52: 134-143.
- Aluru N, Karchner SI, Glazer L (2017) Early Life Exposure to Low Levels of AHR Agonist PCB126 (3,3',4,4',5-Pentachlorobiphenyl) Reprograms Gene Expression in Adult Brain. Toxicol Sci 160: 386-397.
- Aluru N, Karchner SI (2021) PCB126 Exposure Revealed Alterations in m6A RNA Modifications in Transcripts Associated With AHR Activation. Toxicol Sci 179: 84-94.
- Brun NR, Salanga MC, Mora-Zamorano FX, Lamb DC, Goldstone JV, Stegeman JJ (2021) Orphan cytochrome P450 20a1 CRISPR/Cas9 mutants and neurobehavioral phenotypes in zebrafish. Sci Rep 11:23892.
- Brun NR, Panlilio JM, Zhang K, Zhao Y, Ivashkin E, Stegeman JJ, Goldstone JV (2021) Developmental exposure to non-dioxin-like polychlorinated biphenyls promotes sensory deficits and disrupts dopaminergic and GABAergic signaling in zebrafish. Commun Biol 4: 1129.
Additional information about Project 3 Personnel:
Dr. Jennifer Martinez Panlilio
Current position: Postdoctoral Fellow, National Institute of Child Health and Development, National Institutes of Health, Bethesda, MD
Ms. Aliya Hidayat
Current position: Knauss Fellow, NOAA Sea Grant, Washington, DC

Aliya Hidayat
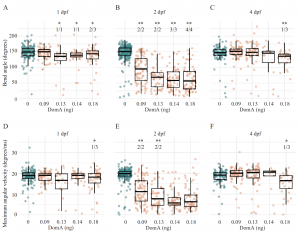
Figure 1. Kinematics of short-latency C-bend (SLC) startles in zebrafish exposed to domoic acid (DA) at 1, 2, and 4 days post-fertilization (dpf). Fish were exposed to different doses of DA at (A,D) 1dpf, (B,E) 2dpf, and (C,F) 4dpf, and startle responses were measured at 7dpf. SLC startle responses were characterized by (A–C) bend angle and (D–F) maximal angular velocity. For details, see [1].
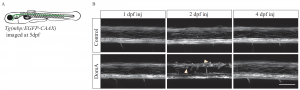
Figure 2. Confocal imaging of myelin sheath structures of zebrafish exposed to domoic acid (DA) at different developmental days (A) Tg(mbp:EGFPCAAX) fish were used to visualize labeled myelin sheaths. (B) Representative images of fish exposed to DA (0.13–0.14 ng) during discrete periods in early development [1–4 days post-fertilization (dpf)], then imaged at 5 dpf using confocal microscopy. Arrows indicate the unusual circular membrane profiles. For details, see [1].
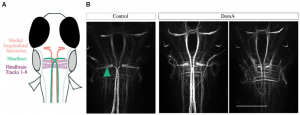
Figure 3. The majority of DA-exposed larvae do not have Mauthner cells but have other hindbrain and midbrain structures. A, Schematic of brains stained with antibody 3A10, which labels Mauthner cells, medial longitudinal fasciculus, and hindbrain axonal tracts. B, Representative images of brains from control and DA-exposed larvae (5 dpf) stained with anti-3A10. Teal arrow points to Mauthner cell. Scale bar = 100 µm. For details, see [2].
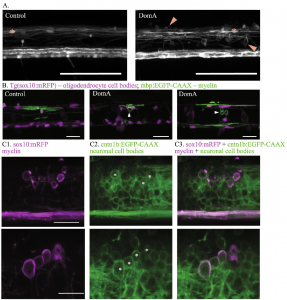
Figure 4. DA exposure at 2 dpf leads to the formation of aberrant circular profiles that may be ectopically myelinated neuronal cell bodies. (A) Tg(mbp:EGFP) × Tg(mbp:EGFP-CAAX) double transgenic line labels both oligodendrocyte cell bodies and myelin sheaths. Asterisks mark oligodendrocyte cell bodies. Arrows label circular myelin membranes. Scale bar = 100 μm. (B) Representative images of mosaically labeled oligodendrocytes in DA-exposed (2 dpf) and control fish following 1–4 cell injections of the reporter construct, mbp:EGFPCAAX into Tg(sox10:RFP) background, imaged at 4 dpf. Oligodendrocyte cell bodies are labeled in red. Arrows mark circular myelin membranes, which are outlined in green. Scale bar = 25 μm. (C) Tg(sox10:mRFP) × Tg(cntn1b:EGFP-CAAX) double transgenic line imaged at 5 dpf. mRFP labels myelin sheaths and oligodendrocyte membranes. EGFP-CAAX labels the membrane of subpopulations of spinal cord neurons. Two examples of DA-exposed double transgenic fish. Circular myelin membranes labeled in RFP (false colored magenta) (C1), neuronal cell bodies outlined in EGFP (C2). Images were then merged (C3). Asterisks mark neuronal cell bodies that are potentially associated with circular myelin membranes in the imaging plane. Top image scale bar = 25 μm., bottom image scale bar = 20 μm. For details, see [3].
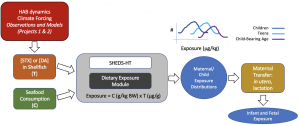
Figure 5. Exposure modeling.
