Analytical Prices and Precision for Water Samples
Price Schedule
The primary goal of this facility is to make helium isotope, tritium, and noble gas measurements available to the U.S. academic research community. Analytical charges are made on a per sample basis and reflect the cost of doing the measurements. Commercial analyses (i.e., for organizations other than educational, government-funded, or non-profit) are also offered, but a higher rate. Please contact us at aseltzer@whoi.edu (with a cc to kcahill@whoi.edu ) regarding scheduling, sample submission, and sampling protocols/requirements. Sample prices below are for calendar year 2025. For planning purposes, consider adding 4% per year on future prices, depending on when you submit the samples. We apologize for the increases but they are driven by inflation and increasing overhead costs.
2025 Prices
We are currently accepting requests for dissolved helium isotope and noble gas abundance measurements in copper tube for routine analysis at 0.1-0.2% precision in copper tube samples. We are not routinely measuring tritium at this point, but please do not hesitate to reach out, as we may consider certain requests for tritium analysis, depending on the scope.
Standard copper tube measurements (He isotopes & He/Ne/Ar/Kr/Xe abundances): $452 per sample
For high-precision (sub per-mil) Ar/Kr/Xe isotope measurements, please see our companion lab site. (We are in the process of integrating the two facilities.)
Note: These prices do not include an approximately 65% Institutional overhead charge (this is related to the WHOI MTDC overhead cost recovery structure, please contact us for the actual rates). Please direct enquiries to aseltzer@whoi.edu with a cc to kcahill@whoi.edu. We define "Non-Commercial" as being U.S. government-funded, academic, or non-profit entity research. Sample acquisition, containers, and shipping costs are borne by the client.
Note: administrative, logistical, and data analysis support required to facilitate analysis is billed separately, at an approximate rate of 1 week of support for the lab manager (K. Cahill) per month of analytical time. (Factoring in down time, we can typically run roughly 80 samples per month.)
Note: samples of unusual size, form factor, requests for fewer than 30 sample analyses, or containing elevated levels of non-atmospheric He, for example from hydrothermal or volcanic sources, will require special handling and analysis at additional "set up" cost due to their variable sizing and non-atmospheric 3He/4He isotope ratio, which may require extra standardization of our instruments. Please contact us to discuss your options.
Good results require good samples, so we have stringent requirements on sampling procedures and materials. We can and will assist you in mounting an effective sampling program and can provide you the appropriate equipment and materials. Please contact Kevin Cahill (kcahill@whoi.edu) for information.
Measurement Precision
Tritium Measurements: Please understand that because of contamination considerations, we only measure "natural level" tritium samples, not samples expected to have elevated concentrations due to, for example, radioactive waste disposal or nuclear reactor leaks. Tritium determinations are made by 3He-regrowth mass spectrometry on water samples ranging from 100 cc to 1000 cc in volume. Samples must be in clean (tritium-free) glass bottles with poly-seal caps, and preferably with an argon head-space. Analytical detection limit is related to sample size: the larger samples are required to obtain a lower detection limit. A 1 liter sample would permit a detection limit of 0.005 TU while a 0.1 liter sample provides only 0.02 TU. Measurement accuracy approaches 1% at higher tritium concentrations, but is limited by ion counting statistics as the concentration approaches detection limit. Turnaround times (the time between sample submission and result reporting) are controlled by a combination of incubation times (minimum 6 months), sample scheduling and mass spectrometer "down-time". Please consult with IGF staff (aseltzer@whoi.edu) regarding your needs.
Helium Isotope and Concentration Measurements: Helium isotope and concentration measurements are made on gas extracted from water samples. Samples are typically taken in copper tubing samplers, which consist of lengths of copper tubing clamped at either end with specially designed clamps. These are extracted in the laboratory into aluminosilicate glass ampoules that are subsequently mounted on the mass spectrometer for analysis. The achievable measurement precision depends on sample size. For example, 45g "normal" water samples, typical measurement precisions are 0.15% for the 3He/4He isotope ratio, and 0.5% by peak-height manometry for total dissolved He. For smaller samples (e.g., 12 g), the accuracy is degraded due to less favorable counting statistics. The table below gives an approximate idea of what to expect. Ancillary measurement of Ne concentrations are usually also made by QMS peak-height manometry to approximately 0.5% accuracy. Our primary standardization is atmospheric He and Ne, and the the 3He/4He isotope ratio is reported in percent deviation from the atmospheric standard. The results are corrected for a slight dependence of the apparent 3He/4He isotope ratio on sample size. Samples with elevated levels of non-atmospheric He (with different 3He/4He isotope ratios and abundances) will require special handling and must be dealt with on a case-by-case basis.
Sample Water Size (g) Expected 3He/4He Precision
45 0.15% to 0.25%
12 0.3% to 0.5%
1 1-2%
Noble Gas Measurements: The quoted noble gas and helium isotope measurement price assumes analysis of 45g samples. Samples are processed on our new noble gas mass spectrometer system (MS3) for both noble gas abundances and the 3He/4He isotope ratio. Extracted gas samples are purified by cryogenically removing water, and by exposure to Pd catalyst (to remove methane) and SAES getters (to remove reactive gases, including hydrogen). The noble gases are subsequently separated by programmed temperature sorption/desorption on two cryogenic traps (one "nude" and one with activated charcoal). The noble gas abundances for He, Ne, and Ar are determined by peak-height manometry using an ion counting triple mass filter QMS to a precision of about 0.1% as measured by reproducibility of standard gases. For Kr and Xe, we use a ratiometric isotope dilution method that provides similar precisions. There is an inherent overall systematic uncertainty associate with standard size of 0.15%. Noble gas accuracy for water samples, as indicated by replicate analysis is slightly worse (see table below for 45 gram samples), and is likely related to sampling and gas extraction effects. We also expect that reproducibility for 12 gram samples will be as large as 0.25%. Helium isotopes are measured using a magnetic sector mass spectrometer to 0.1 to 0.15% for 45 gram water samples.
Noble Gas Gas Reproducibility Water Reproducibility
He 0.10% 0.20%
Ne 0.10% 0.20%
Ar 0.10% 0.20%
Kr 0.10% 0.20%
Xe 0.10% 0.20%
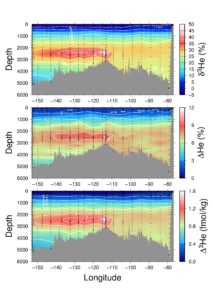
The zonal distribution of helium isotopes along approximately 15 S in the Pacific, taken during the GEOTRACES GP16 EPZT expedition in 2013. Note the plume of hydrothermal helium at ~2500 m extending westward from the East Pacific Rise. It is evident not only as an isotopic anomaly (expressed in % in the uppermost panel), but also as a concentration anomaly (the saturation anomaly expressed in % in the middle panel). From this we can calculate the absolute amount of excess helium-3 (expressed in fmol/kg in the lower panel).
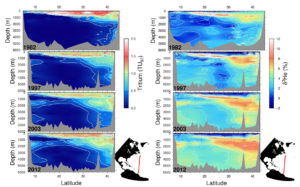
A multidecade time series (from 1982 to 2012) of tritium (left) and helium-3 (right) in the subtropical North Atlantic along approximately 53 W. The tritium is reported in Tritium Units decay corrected to a common time (1997), and the helium isotope ratio anomaly is expressed in % relative to atmospheric standard. Note the progressive invasion of tritium into the deep waters and its dilution in the shallow thermocline region. Note also the buildup of helium-3 in intermediate and deep waters associated with tritium decay.
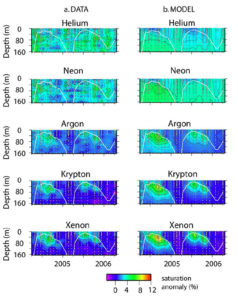
A two year time series of noble gas saturation anomalies at Bermuda, data-model comparison. This research was done by Rachel Stanley.