Microbial production
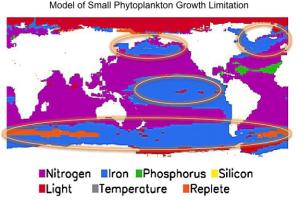
Trace metals such as iron, cobalt, nickel, copper, and zinc are essential for metabolic pathways common to all marine phytoplankton and bacteria. Due to the poor solubility of most metals in seawater, however, metal concentrations are often exceedingly low and limiting for the growth of marine phytoplankton. For example, large areas of the Equatorial Pacific, Northwest Pacific and Southern Oceans are characterized by low intrinsic productivity due to iron limitation (Fig. 1). Ocean fertilization with iron has been proposed as one way to mitigate the effects of global climate change, but will any of iron added be available to phytoplankton? One mechanism by which microbes acquire iron in low iron environments is through the production of siderophores (low molecular weight compounds that form very strong complexes with iron). Siderophore producing bacteria have specialized mechanisms for recovering siderophore-bound iron, giving them a strong competitive advantage in iron limiting environments. If siderophores are a large fraction of iron binding ligands in seawater, we expect them to have specific structures related to known siderophores.
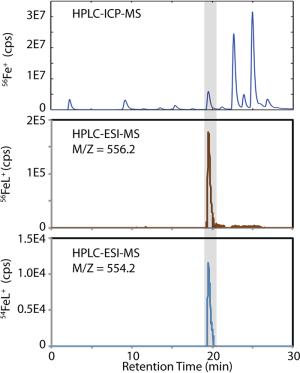
Electrochemical measurements show that as much as 80 to 99.9% of trace metals in the ocean are complexed by organic ligands, yet little is known about ligand chemical composition. Determining the identity, source, and biological activity of trace metal organic ligands is a critical step towards an understanding of the interaction between climate, anthropogenic driven environmental change and microbial ecosystems. Organic speciation of trace metals in environmental systems is an emerging field made possible by recent analytical advances in mass spectrometry. In collaboration with Jess Fitzsimmons in Ed Boyle’s lab at MIT we have developed a tandem high pressure liquid chromatography-inductively coupled plasma mass spectrometer (HPLC-ICP-MS) interfaced with an electrospray ionization mass spectrometer (ESI-MS) that allows us to detect, quantify, and purify organic-metal compounds for structural characterization (Fig. 2). The goals of our work are to characterize the composition of trace-metal organic complexes, understand how and why they vary between ocean biomes, and identify the organisms that are responsible for their production. Finally, we wish to measure how trace metal organic ligands impact iron acquisition by microorganisms.
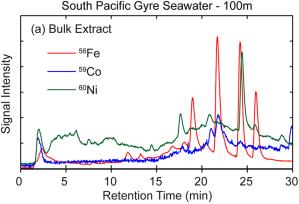
In collaboration with Penny Chisholm’s lab at MIT we have focused our efforts on the production of iron binding organic ligands in pure laboratory cultures of marine cyanobacteria (Prochlorococcus sp. and Synechococcus). These cyanobacteria represent more than half of global marine primary productivity, and we are using them as model systems to study trace metal acquisition in iron-limited environments (Fig. 3). We find marine cyanobacteria and bacterioplankton produce suites of iron and cobalt ligands that we are working to identify (Fig 4.). We find an equally diverse suite of ligands in all the seawater samples we have screened to date (Fig. 5), including sites where prochlorococcus and synechococcus dominate the phytoplankton community. In the coming years we will use a combination of high resolution mass spectrometry and microcapillary nuclear magnetic resonance spectroscopy to determine the structures of these ligands and better understand their role in iron and cobalt acquisition.