Gasoline, Whole Foods apples, and everything else “organic”
What does it mean for a chemical to be organic? This question itself might confuse some readers. Outside of chemistry labs and classrooms, many people think of the word “organic” in the context of describing food that has been grown without synthetic pesticides. Similarly, many people think of synthetic pesticides as being synonymous with the word “chemical,” so the idea of a “chemical” that is also “organic” would appear to be an oxymoron. You would have reason to be confused: The way we use scientific words to talk about food sometimes results in ludicrous misrepresentations. I recently bought some basil that was packaged with the label “biologically grown.” How one would grow basil non-biologically is a mystery to me, but I welcome suggestions.
In fact, “organic” has a very different meaning in a chemistry lab than it does in a grocery store. For a chemical to be organic, all it needs is to be made up of carbon atoms (represented by C) with some hydrogen atoms (represented by H) attached to them. It can also have other atoms attached, most commonly oxygen (O) or nitrogen (N), but the backbone of an organic molecule is always C and H. The simplest organic molecule is methane, which is just a carbon atom bonded to four hydrogen atoms. In the drawing of methane below, the lines represent the chemical bonds between C and H:
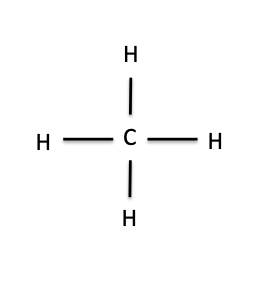
Methane
The structure of methane can tell us some important things about how organic molecules work and why they’re important enough to have their own chemical taxonomic class. As you notice in the picture above, carbon has four bonds to hydrogen. There’s a rule in chemistry that every element can make only a certain number of bonds to other elements, based on where they are located on the periodic table. Carbon is always in its most stable form when it makes four bonds to other atoms: It will never make more than that. If you take away one of those bonds, so that only three are present, the carbon atom will freak out and do whatever it can to restore the missing bond; it’s too unstable to exist for long without it. This is what often drives a chemical reaction. For example, if you throw some methane together with a chlorine gas and expose it to high-energy light, a chlorine radical (a very reactive form of the chlorine atom, drawn like this: Cl·) will form. The chlorine radical will break one of the bonds between C and H, leaving behind a reactive methyl radical. The unstable methyl radical will then steal a chlorine atom from the chlorine gas to satisfy its need for a fourth bond. We can draw that reaction like this:
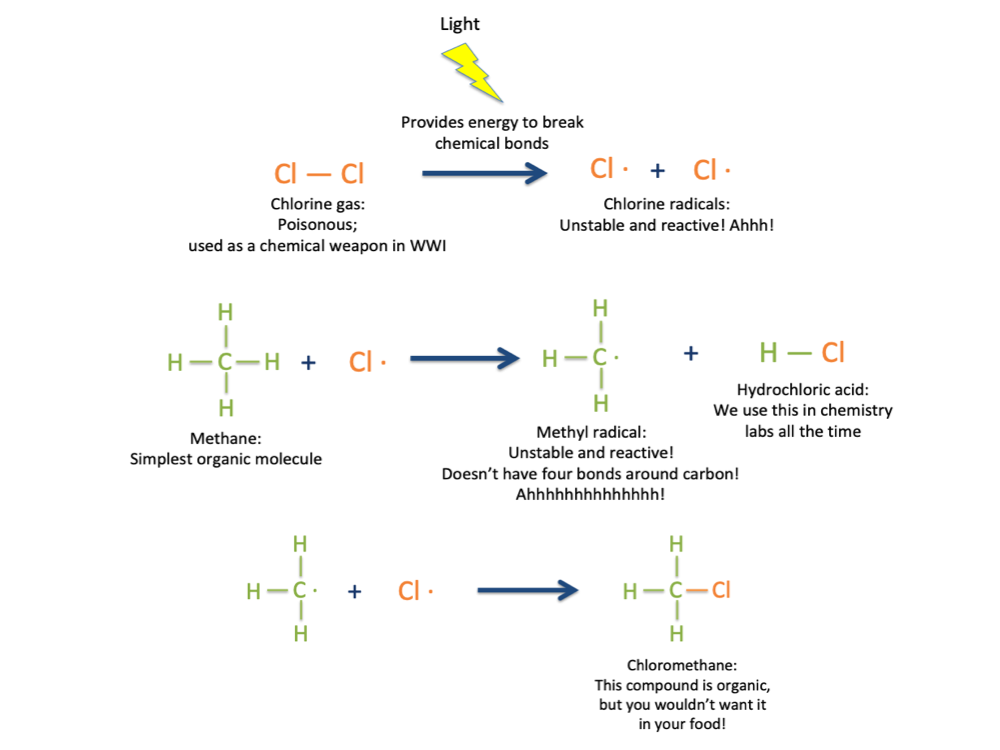
The product molecule, chloromethane, occurs naturally on the sea surface, is produced synthetically for use by chemists in the lab, is toxic at high concentrations, and is also organic, because it has C-H bonds in it [1-2].
The fact that carbon is in its most stable form when it has four bonds to other atoms turns out to be pretty important. It allows carbon to form long chains in which each C is attached to at least one other C and the remaining bonds are satisfied by H atoms. For example, if, in the previous example, we replaced one of the H’s on methane with another CH3 group (called a methyl group) we would end up with this molecule, ethane:
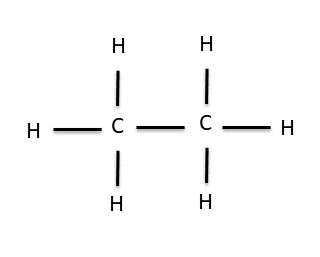
Ethane
We can keep adding methyl groups in this manner to get longer and longer chains of carbons and hydrogens. These chains are called hydrocarbons. The hydrocarbon below, with eight carbons in the chain, is called octane. The word octane might sound familiar; it is the major component of gasoline.
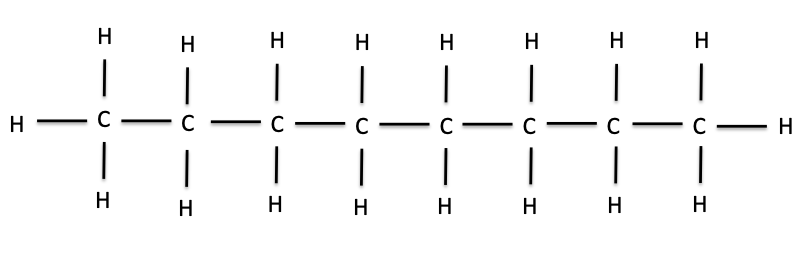
Octane
When we get to super long hydrocarbon chains like octane, it takes a lot of time to write out all of the C’s and H’s and the bond lines between them, so organic chemists use a short-hand called bond-line structures. In bond-line structure form, octane looks like this:
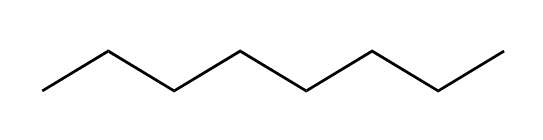
Bond-line structure of octane
The zig-zag, pictogram language of bond-line structures scares many an incoming organic chemistry student, but you can learn how to read these structures quickly and easily. In the bond-line structure above, each corner and each end point is a carbon atom. The zig-zag lines indicate bonds between carbon atoms, and the bond lines to H atoms are not drawn in. How do we know how many H’s are present, then? When reading bond-line structures, we assume that every carbon atom is in its most stable form, with four bonds to other atoms. So if a carbon is in the middle of the chain with two bonds to other carbons, we know that there must also be two H’s attached to that carbon. They just aren’t drawn in. If the carbon is at the end of the chain, with only one bond to another carbon, that means it must have three H’s attached. The diagram below might help if you’re still confused:
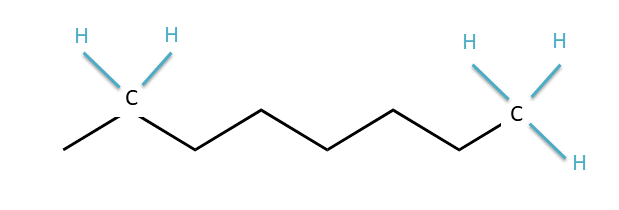
Locations of implicit hydrogen atoms in a bond-line structure
Straight-chain hydrocarbons like octane are the simplest kind of organic molecule. But because carbon can make four bonds, we can make other geometric arrangements of C and H as well. By replacing C-H bonds with new C-C bonds at different positions, we can make branched hydrocarbons, and rings:
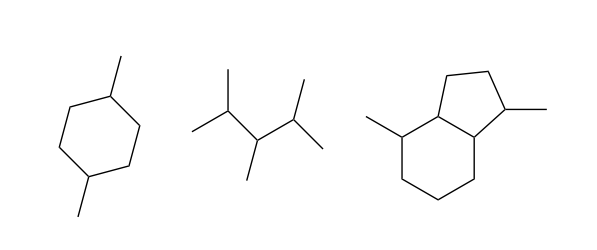
Organic compounds with branches and rings
We can also functionalize, which is an organic chemistry vocabulary word for replacing C or H atoms in a hydrocarbon with other atoms, such as O or N. Alternatively, we can make double or even triple bonds between carbon atoms, reducing the number of C atoms to other molecules:
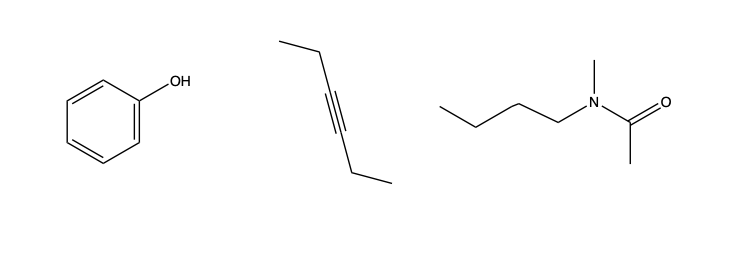
Functionalized organic compounds
When organic chemists do reactions in a lab setting, they break bonds, swap out atoms, and change the geometric arrangement of molecules like this. It’s like playing with Legos, except for the fact that the Legos are on the order of 10-12 meters in size and are also the fundamental building blocks of all of our material world. It’s pretty cool. Also pretty scary, to realize that we have power to alter our material world at the most fundamental level, making new molecules that perhaps have never existed before in nature.
Why do we care about the fact that carbon can make extended molecules like this? We care because it’s not very common. Most other elements don’t do this, in large part because most other elements don’t make four bonds to other atoms. Oxygen, for example, only makes two bonds to other atoms. Hydrogen only makes one. When these elements bond to themselves, they only make measly two-atom gas molecules, O2 and H2. But carbon’s four bonds allows it to build organic molecules of incredible size and diversity, some gases (like methane), some liquids (like octane) and some solids—like sugars, fats, and proteins, and also plastics of every sort.
This brings me to the first reason why it’s worthwhile understanding what “organic” means in a chemical context. Organic molecules are the basis of life as we know it, which is why in common talk the word “organic” has an association with being wholesome or natural. This is where the idea of “organic” food comes from. Hopefully you can see at this point though that this association is unfortunate from a scientific perspective. Organic molecules do make up much of our natural world; they also are the basis of most of the synthetic substances we use too. For me, the point is that the distinction between natural and synthetic is often unhelpful when thinking about the role of organic molecules in our world, whether benign or toxic. It’s more helpful to look at the chemical structures themselves to evaluate their behavior in the world around us.
There’s a second reason why it’s worthwhile walking through life knowing the definition of organic chemistry, which is that it reminds us what much our material world is truly made of. Here is what is beautiful about chemistry: It is not actually about something abstract, intangible, foreign. Certainly bond-line structures are an abstraction, the dual wave-particle qualities of electrons make them intangible, and I think there are very few people who would not find molecular orbital theory foreign. But in fact, these abstract, intangible, foreign-seeming ways we have of communicating chemistry are all a means of explaining the most concrete, tangible, and local things in the world around us. When I walk outside of the building I work in, tired from hours of reading scientific articles, I am reminded with amazement that those organic molecules I was just reading about are what make up the trees and grass around me, that those geometric arrangements of mostly C, H, N, O have somehow assembled themselves into materials as diverse as tree bark and pollen and butterfly wing. I think then of organic chemistry bond-line structures, and the other ways we have of representing chemical structures and behavior, as a kind of Impressionist painting of the underpinnings of our material world, our way of grasping a reality so startlingly complex and beautiful and specific that it is impossible to render in the detail it deserves.
References:
[1] Singh, H. B.; Salas, L. J.; Shigeishi, H.; Scribner, E. Atmospheric Halocarbons, Hydrocarbons, and Sulfur Hexafluoride: Global Distributions, Sources, and Sinks. Science. 1979. [2] Public Health Statement for Chloromethane. Agency for Toxic Substances & Disease Registry. CDC. Dec. 1998. https://www.atsdr.cdc.gov. Accessed 4 Aug. 2019.Acknowledgments:
Thanks to Anna Walsh and Briana Prado for providing their feedback on a draft of this post.