Neel Aluru, Mark Hahn, John Stegeman, Sibel Karchner (WHOI), and Wendy Heiger-Bernays (Boston University School of Public Health
The overarching objective of this project is to elucidate the fundamental cellular and molecular mechanisms of toxicity associated with developmental exposure to harmful algal bloom (HAB) toxins. Humans are exposed to HAB toxins at levels that are below the federal regulatory limits and do not elicit overt signs of toxicity, but have been shown to cause long-term neurobehavioral deficits in animal models and people. However, the cellular and molecular mechanisms of developmental neurotoxicity and the potential human health implications of developmental exposure to these low levels of HAB toxins are not well understood.
Our studies in zebrafish embryos, a well-established model in developmental biology and toxicology, suggest that exposure to the HAB toxins domoic acid (a glutamate receptor agonist) and saxitoxin (a voltage-gated sodium channel blocker) affects multiple cell types (neuronal and non-neuronal) in the brain that are critical for proper neural development and function. However, it remains to be determined which cell types are the initial targets of toxin exposure. In addition, whether exposure to HAB toxins affects differentiation of multipotent progenitor cells into neuronal and glial cell types is unknown. Understanding of the cell-specific effects of exposure to HAB toxins on the developing nervous system and the cumulative effects of HAB toxins and toxicants is essential.
The central hypothesis of the proposed research is that exposure to HAB toxins during early development alters various neuronal and glial cell types independently, leading to cell-type specific transcriptional changes, ultimately contributing to altered neurobehavioral outcomes. We propose to test this hypothesis using two complementary model systems: zebrafish, an established model organism for characterizing molecular, cellular, and behavioral changes in vivo, and human iPSC-derived 3D brain systems in vitro for elucidating the effects of toxins on differentiating human neural cells. The specific aims of the proposed research are:
Specific Aims:
Aim 1: Identify cellular targets of HAB toxins using single-cell transcriptomic signatures.
We will test the hypothesis that developmental exposure to HAB neurotoxins that act by distinct mechanisms—domoic acid, saxitoxin, or anatoxin-a—causes cell-specific effects on neuronal and glial progenitor cells that alter cell-fate and differentiation. As brain is a highly heterogenous tissue, previous studies using bulk RNA-seq could not attribute gene expression changes to a specific cell-type. Using single cell RNA-seq, we will quantify cell-specific gene expression signatures and infer gene regulatory networks that are impacted by exposure.
Aim 2: Assess the effects of early life exposure to HAB toxins on neural differentiation and proliferation.
Using human iPSC-derived 3D brain systems that contains neurons, astrocytes and oligodendrocytes, we will test the hypothesis that HAB toxins affect the differentiation and proliferation of neural progenitor cells. We will investigate the effect of domoic acid on differentiation of oligodendrocytes and neurons and on myelination. Informed by Aim 1 results, we will determine the effects of saxitoxin and anatoxin-a on progenitor cell differentiation. The results will provide insight into potential effects of HAB toxin exposure on human neurodevelopment, and the mechanisms involved.
Aim 3: Determine the interactive effects of persistent organic pollutants and HAB toxins.
We will test the hypothesis that developmental or preconception exposure to neurotoxic polychlorinated biphenyls (PCBs) affects the later-life sensitivity to HAB toxins and that the differences in sensitivity vary across the lifespan. Zebrafish embryos will be exposed to low levels of neurotoxic PCBs and then raised in clean water to adulthood. These juvenile and adult fish will be exposed to environmentally relevant levels of HAB toxins and neurobehavioral responses will be assessed. Similarly, the effects of adult preconception exposure to PCBs on larval sensitivity to HAB toxins will be determined.
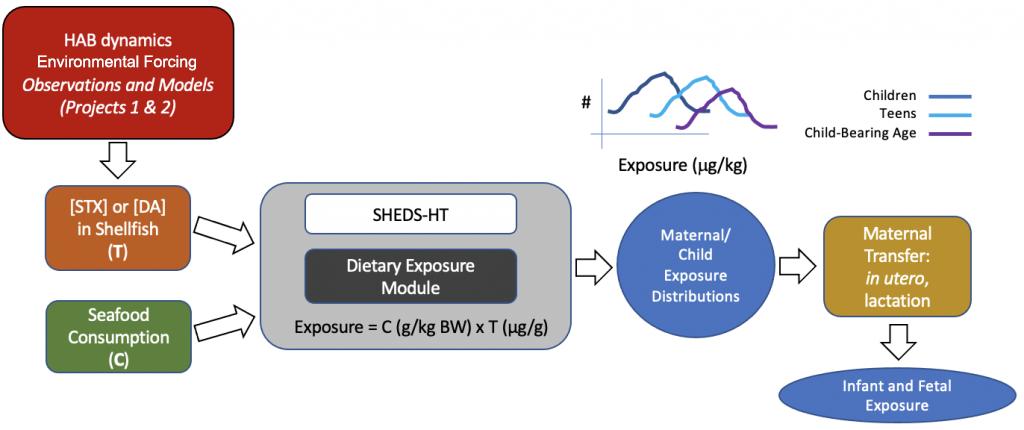
Aim 4: Modeling exposure of susceptible human subpopulations to HAB toxins.
We will assess human exposure to domoic acid during susceptible windows of development determined in our prior studies in zebrafish embryos. We also will collaborate with Projects 1, 2, and the CEC using coupled biophysical oceanographic and exposure models to predict human exposure to HAB toxins domoic acid and saxitoxin.
Integration
The proposed research will incorporate information on HAB bloom dynamics (Project 1) and models (Project 2) to better understand human exposure to HAB toxins and how it may change, in consultation with stakeholders (CEC).