A silent, sunlit shift in the Arctic carbon cycle
I wrote previously about how sunlight exposure can influence the fate of oil slicked on the surface of the ocean after an oil spill. In this post, I’ll share how photochemical reactions, or reactions caused by sunlight exposure, are also an important player in carbon cycling and climate change.
What is carbon cycling, and why should you care?
I happen to be a major fan of the X-Files, but at the risk of making a sweeping statement that would offend the sensibilities of Fox Mulder, I will assert that life as we know it, here on Earth, requires the production of organic carbon. Your body and mine, and that of every other living thing on Earth, from bacterium to blue whale, is a construction of molecules of organic carbon. We are made of a diverse array of these molecules, from proteins to sugars to lipids, but these compounds share a carbon-hydrogen backbone that defines them as organic. You can see some examples of those chemical structures here:
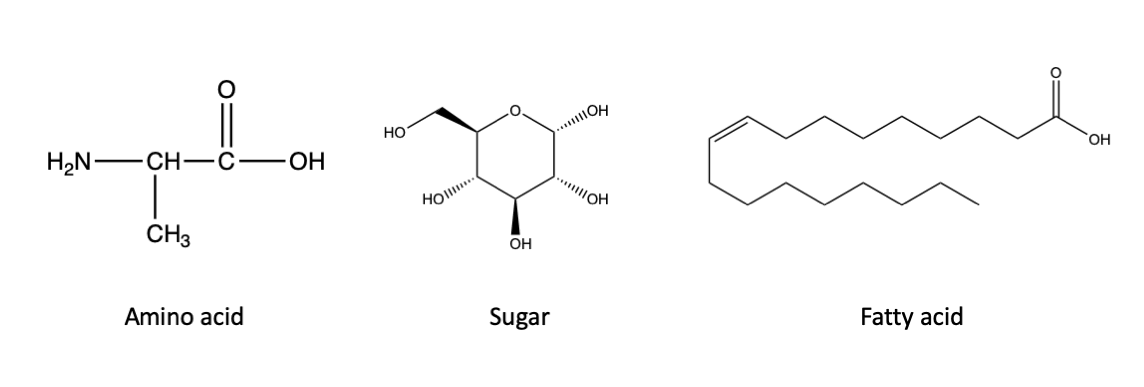
Left, an example of an amino acid, which are the building blocks of proteins. Middle, an example of a sugar. Right, an example of a fatty acid, a subset of lipids. All of these compounds are organic, as indicated by the carbon-hydrogen backbone of the molecules. Click here for a refresher on what “organic” means to a chemist and how to read these chemical structures.
Where do our bodies get the carbon atoms (along with some hydrogen, oxygen, nitrogen, etc.) to build these organic molecules? You might remember that matter cannot be created nor destroyed, so we can’t just invent new carbon atoms. Instead, we have to eat food containing organic chemicals that our bodies break down and reconfigure into the molecules we need to build bones, blood, muscles, etc. In turn, the organic molecules in our food can be traced back originally to plants. Those plants generated organic carbon molecules by photosynthesis, a process which traps gaseous carbon dioxide from the atmosphere and converts it to organic form. We return carbon to the atmosphere when our bodies respire, breathing out carbon dioxide.
This cycle of photosynthesis, food consumption, and respiration is how each and every one of us interacts with the “global carbon cycle,” or the transfer of carbon atoms among various parts of the environment, such as the atmosphere, oceans, and biosphere (life). We are all conduits for the chemical transformation of carbon. There are many processes that affect the global carbon cycle, including not only photosynthesis and respiration, but also volcanism, the dissolution of carbon dioxide into the ocean, and the burial of carbon in the deep ocean. In the past two hundred fifty odd years, another process has had a major impact on the global carbon cycle: the burning of fossil fuels, which has added a major slug of carbon dioxide to the atmosphere and led to climate change [1].
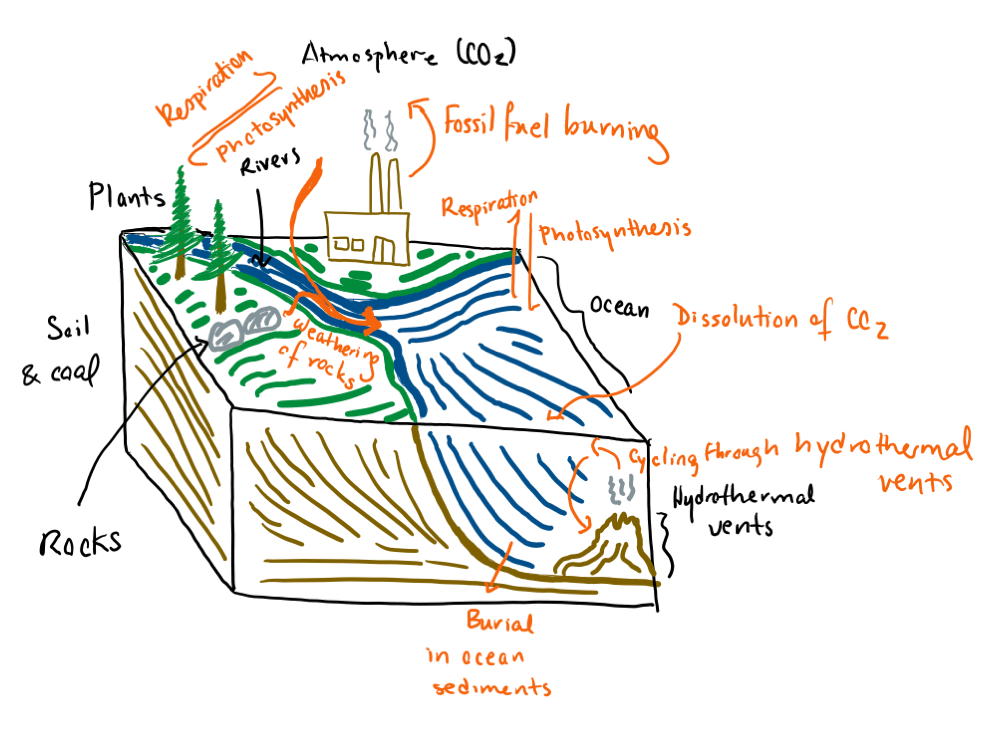
The global carbon cycle. Reservoirs of carbon (ex: the atmosphere, rocks) are labeled in black. Processes affecting the movement of carbon from one part of the environment to another (ex: burial in ocean sediments) are labeled in orange. This is a simplified diagram that doesn't include every possible reservoir or transfer process. For more information, see Emerson & Hedges [1].
Photochemistry and carbon cycling
Recent research has added another orange arrow to the diagram above: photochemistry. In a 2014 article in Science, researchers found that chemical reactions caused by sunlight were a major player influencing the fate of organic carbon released from soils in the Arctic [2]. Furthermore, photochemistry may become a more important process influencing the fate of organic carbon as soil carbon that was previously locked up in frozen permafrost for thousands of years is released due to warming temperatures [2].
So, what is this soil carbon, and how does it interact with sunlight? As the composters among us know, soils are a major repository of carbon-containing organic molecules, which come from the decomposition of poop and dead stuff. This stock of organic carbon is important in soils because it provides the nutrients needed for plants to grow (Remember, nothing is created or destroyed! Nature recycles everything!). However, not all that organic carbon stays in soils. Some of it leaches into groundwater—just like organic molecules in a teabag leach into a mug of water—and gets transported to streams, rivers, lakes, and oceans. Once dissolved in surface waters, the organic carbon is exposed to microbes, which can further degrade the organic carbon and respire it into CO2. The organic carbon is also, at this point, exposed to sunlight.
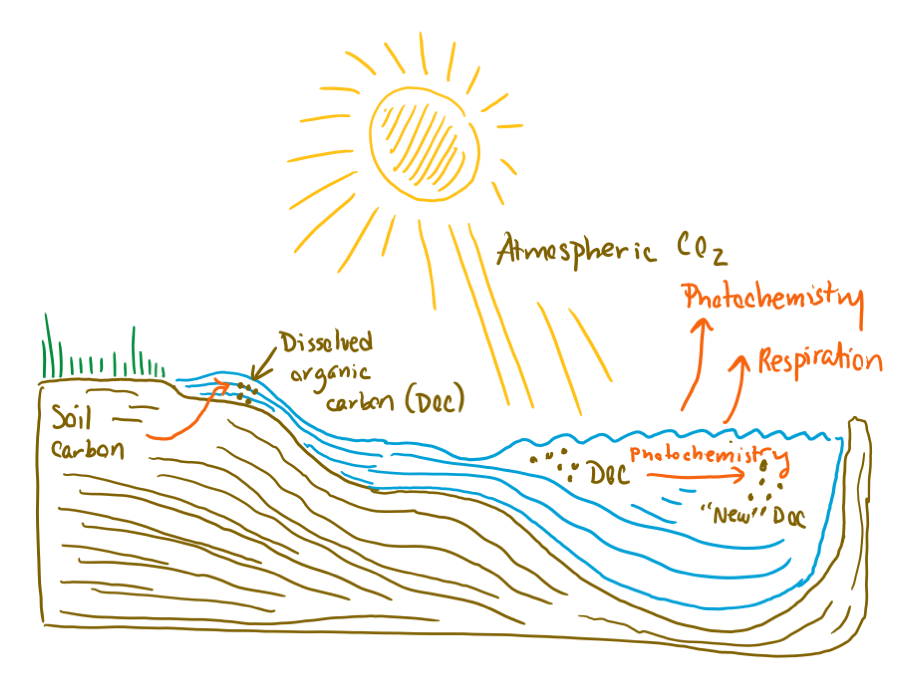
Diagram showing the fate of soil carbon in inland Arctic waters. "New" DOC is dissolved organic carbon that is transformed by photochemistry into new organic molecules (as opposed to converted to CO2, which is released to the atmosphere).
When the organic carbon is exposed to sunlight, energy from the light causes chemical reactions that can alter the organic molecules present. There are two possible outcomes when this happens. The first possibility is that the original organic molecules are converted to different organic molecules with different chemical properties. You might think of this type of reaction as a “sunburn” affecting the organic carbon. The second possibility is that the original organic molecules are converted to carbon dioxide and released from surface waters into the atmosphere.
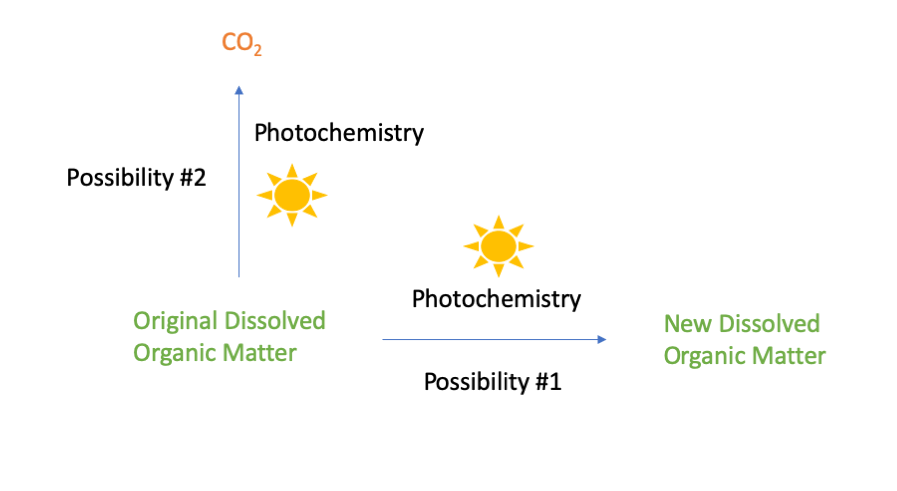 Pathways for the transformation of dissolved organic matter by photochemistry.
Pathways for the transformation of dissolved organic matter by photochemistry.
Until recently, photochemical transformations of carbon were typically assumed to be minor compared to biological respiration [3], but we now know differently: In some circumstances, photochemistry is far more important than biology in transforming organic carbon. For example, as I’ve presented elsewhere, photochemistry is a key process influencing the fate of slicked oil on the surface of the ocean following an oil spill [4]. Similarly, the researchers behind the 2014 Science article found that photochemistry is the dominant process influencing the fate of organic carbon released from Arctic soils into lakes and rivers. Specifically, the researchers found that the vast majority (70-95%) of dissolved organic carbon transformed in Arctic lakes and rivers was transformed by photochemistry rather than by biology. In different water bodies, the relative importance of photochemical conversion to new organic molecules (possibility 1, above) versus photochemical conversion to CO2 (possibility 2) varied. But on average, they found that photochemistry was directly responsible for a third of all CO2 emissions from Arctic inland waters. [2]
Potential climate change amplification
The idea that burning fossil fuels releases CO2 to the atmosphere, and that this CO2 in turn causes higher global temperatures, is familiar to most people. However, regular non-scientists may be less aware that this initial release of CO2 can disrupt the carbon cycle in other ways that may cause other environmental problems and even amplify climate change. The photochemical processing of organic carbon from Arctic soils is one such example: Permafrost soils in the Arctic currently store about twice the amount of carbon present in our atmosphere [5]. Right now, most of that carbon can’t transform into CO2 because it is frozen. But our climate is warming. Warmer temperatures can melt those frozen soils and release the organic carbon stored within them [6]. And, as explained above, once the organic carbon is released to lakes and rivers, sunlight and biology can transform it into CO2. Which can then lead to more CO2 in the atmosphere and even more climate warming. We call this a positive feedback loop.
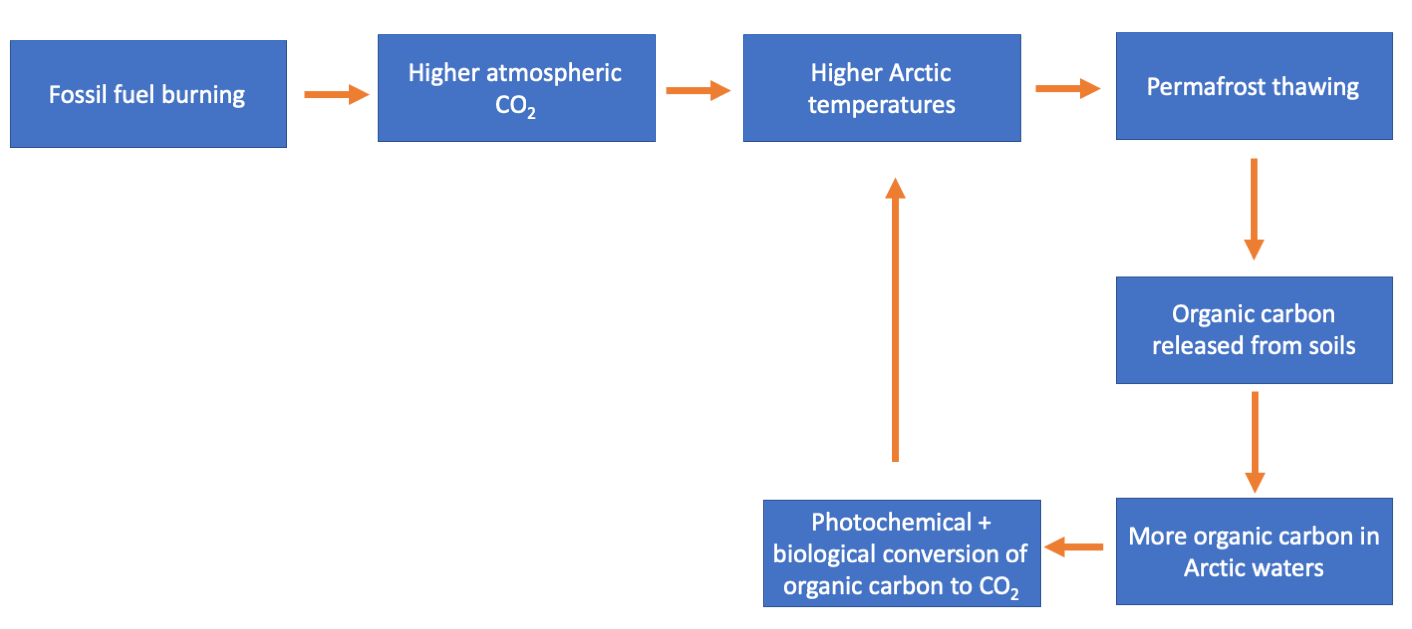
Potential climate change positive feedback loop triggered by permafrost thawing.
If this sounds like a dire situation, it is. There’s no sugarcoating that. A positive feedback climate warming loop like what I’ve described above is a real and scary possibility. What this means is that we need to know a lot more about the processes that would control such a feedback loop. Specifically, we need to know a lot more about the photochemistry that transforms organic carbon released from Arctic soils. Understanding when and how photochemistry is important in transforming organic carbon to CO2 will allow us to put some constraints on this feedback loop and figure out how much of a problem it poses.
References:
[1] Emerson, S.R. & Hedges, J.I. Chemical Oceanography and the Marine Carbon Cycle. Cambridge University Press. 2008. [2] Cory, R. M.; Ward, C. P.; Crump, B. C.; Kling, G. W. Sunlight Controls Water Column Processing of Carbon in Arctic Fresh Waters. Science (80-. ). 2014, 345 (6199), 925. [3] Kling, G. W.; Kipphut, G. W.; Miller, M. C. Arctic Lakes and Streams as Gas Conduits to the Atmosphere: Implications for Tundra Carbon Budgets. Science (80-. ). 1991, 251 (4991), 298–301. https://doi.org/10.1126/science.251.4991.298. [4] Ward, C. P.; Sharpless, C. M.; Valentine, D. L.; French-McCay, D. P.; Aeppli, C.; White, H. K.; Rodgers, R. P.; Gosselin, K. M.; Nelson, R. K.; Reddy, C. M. Partial Photochemical Oxidation Was a Dominant Fate of Deepwater Horizon Surface Oil. Environ. Sci. Technol. 2018, 52 (4), 1797–1805. https://doi.org/10.1021/acs.est.7b05948. [5] Tarnocai, C. Soil Organic Carbon Pools in the Northern Circumpolar Region. 2012, Vol. 23. [6] Zhang, Y.; Chen, W.; Riseborough, D. W. Temporal and Spatial Changes of Permafrost in Canada since the End of the Little Ice Age. J. Geophys. Res. Atmos. 2006, 111 (22). https://doi.org/10.1029/2006JD007284.