On sunlight, oxygen isotopes, and oil spills
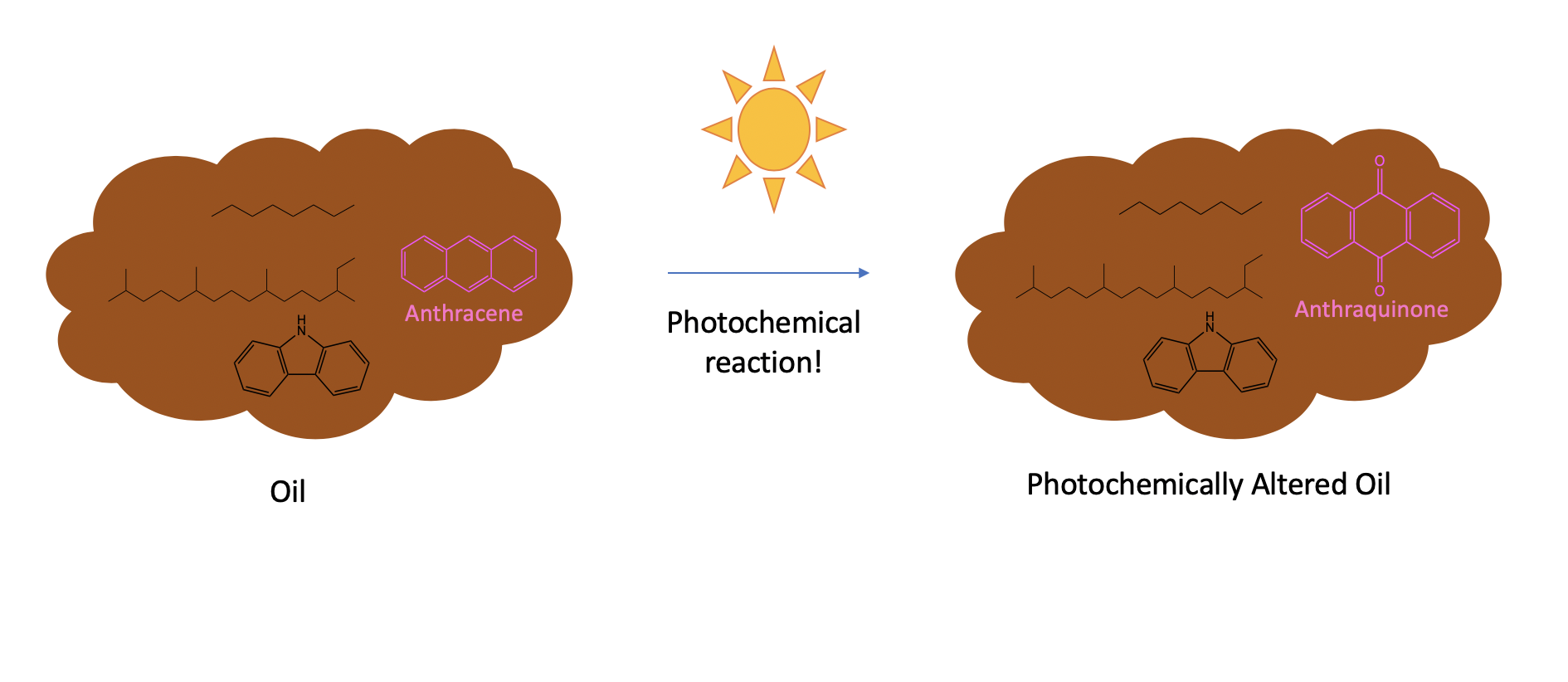
Sunlight exposure alters the composition of oil by photochemical reactions. Note the transformation of anthracene, a compound which does not contain oxygen (“O”), to anthraquinone, which does.
For many months now, I have been rereading the same scientific article, working (hopefully) towards a better understanding of it. The article is the story of some detective work done to better understand the chemical changes that occurred when oil from the Deepwater Horizon oil spill was exposed to sunlight. You can find the article yourself in Geophysical Research Letters, “Oxygen Isotopes (δ18O) Trace Photochemical Hydrocarbon Oxidation at the Sea Surface” [1].
This story starts with some forgotten organic molecules at the bottom of the sea. They were so completely forgotten that for millions of years they were trapped beneath sediment and even rock. These molecules were once a part of the living, breathing world. They were bits of living ocean creatures, cells of phytoplankton or zooplankton, shed skin from a whale or poop from a fish. They floated originally in the surface ocean, basking in sunlight. But a wave caught them crosswise at some point; they were pulled down under; they sank out of the light, down into the cold, salty deep. At the bottom of the ocean, these organic molecules were compressed by layers of sediment and heated by the earth below them, causing chemical reactions that converted the organic molecules to something new—the mixture of hydrocarbons and more complex compounds that we call oil. Millions of years later, someone found these forgotten molecules and began siphoning them out of the ocean floor, up thousands of meters to the surface, where the Deepwater Horizon drilling rig awaited them. This all went as planned until April 20, 2010, when an explosion on the rig broke the pipeline to the oil well below and started the Deepwater Horizon spill. In a previous post, I created a chemical fate diagram (below) that shows what happened to all that spilled oil. Some of it remained in the water column, some of it deposited onto the ocean sediments, and some of it, about 13% floated up to the surface and formed oil slicks [2].
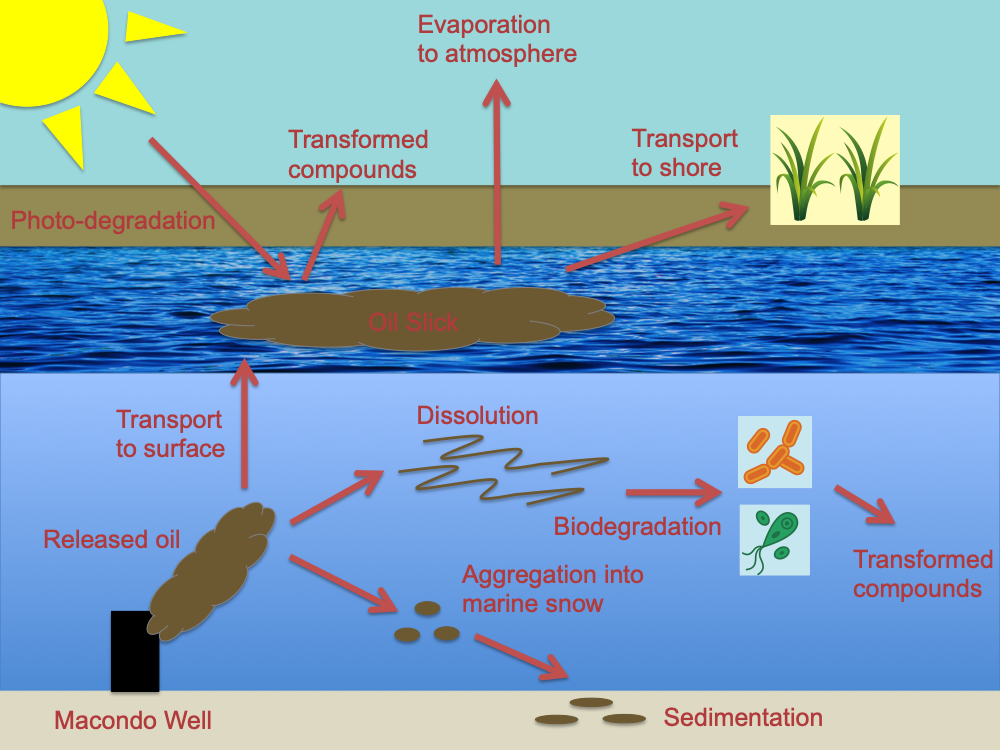
Chemical fate diagram for the Deepwater Horizon oil spill
When this oil reached the surface, it was exposed to sunlight for the first time after millions of years of darkness, and we know from previous work that the light exposure caused rapid photochemical reactions that transformed the slicked oil [3]. In general, we also know that these reactions caused the oxygen content of the oil to increase. For example, sunlight exposure causes a compound like anthracene, which has no oxygen in its structure, to transform into anthraquinone, which has two oxygen atoms [4]:

A reminder: When you see organic chemicals drawn with a bunch of hexagons and zig-zags, as above, each corner represents a carbon atom attached to 1-3 hydrogen atoms. No other elements are present unless they are explicitly drawn in, as the oxygen (“O”) atoms are drawn in anthraquinone. To learn how to read bond-line structures, see my previous post here.
A lot of questions remain, however: What did the oil react with, i.e., where did that new oxygen in the transformation products come from? Can we be sure that the changes we see were actually caused by sunlight as opposed to something else, like biological activity or the preferential evaporation of chemicals that don’t contain oxygen? These questions are important because we know that photochemically altered oil has different properties from the original spilled oil and so has different ecological effects and might require different cleanup tools. We can plan better for how to deal with this transformed oil if we have a way to positively identify the occurrence of photochemistry and quantify its impact compared to other processes.
The researchers from in the GRL paper developed a new tool to identify and trace photochemical transformations of oil by taking advantage of a property called δ18O (“δ” is the Greek letter pronounced “delta”). This property defines the relative abundance of two different types of oxygen in oil: 18O and 16O. These two types of oxygen differ in their atomic masses: 16O, the more common form, weighs 16 atomic mass units, while 18O weighs 18 atomic mass units. These two different forms of oxygen, which are called “isotopes” of oxygen, are otherwise identical and behave the same way in every situation except situations where mass becomes important. For example, the heavier form of oxygen can form stronger bonds than the lighter form. But the lighter form can diffuse (or move around) more quickly, which can cause it to undergo certain chemical reactions more quickly. The different behaviors of the isotopes cause various materials to have different relative amounts of the two, which is measured by the value δ18O in per mil (per mil is like percent, but out of 1000 instead of 100). For example, atmospheric oxygen has a δ18O of 24.6 per mil, while ocean water has a δ18O of 0 per mil, meaning that atmospheric oxygen has relatively more of the heavy 18O isotope compared to ocean water [5]. What’s so crazy and cool is that differences in δ18O are all caused by that initial mass discrepancy between 18O and 16O, which is a difference of only about 3.35 x 10-27 kg. You might imagine that 18O atoms are like a couple of slightly heavy softballs in a huge pile of slightly lighter baseballs; this difference in mass is enough to cause major changes in their environmental behavior.
In the GRL study, the researchers discovered that the δ18O of oil changes after it is exposed to sunlight. The original Macondo oil, they found, had a δ18O of about -0.6 per mil, reflecting a low abundance of the heavy 18O. In contrast, slicked oil that was exposed to sunlight for less than week had a δ18O of 7.2 per mil. This is a big difference that reflects a significant change in the chemical characteristics of the oil! What this means is that the δ18O measurement is quite sensitive to picking up the signal of photochemistry and can be used to detect the extent of this process in the environment.
What is the relevance of this study in the “real world” outside of the lab? This technique could be used in future oil spills (which we hope won’t happen, but it’s best to be prepared …) to identify photochemically altered oil, or to predict when and how quickly oil gets transformed by sunlight, so that spill responders can adjust the response tools they’re using. As mentioned above, photochemically altered oil doesn’t behave the same way as the original oil. A recent study found, for example, that chemical dispersants, which were applied after the Deepwater Horizon spill, are not effective at breaking up slicks of photochemically altered oil [6]. Better understanding when and how quickly oil gets transformed by sunlight can help spill responders decide if applying dispersants in a given situation makes sense, or else if it would be a waste of resources that would unnecessarily introduce more anthropogenic chemicals to the ecosystem.
References
(1) Ward, C. P.; Sharpless, C. M.; Valentine, D. L.; Aeppli, C.; Sutherland, K. M.; Wankel, S. D.; Reddy, C. M. Oxygen Isotopes (δ18O) Trace Photochemical Hydrocarbon Oxidation at the Sea Surface. Geophys. Res. Lett. 2019, 0 (0). https://doi.org/10.1029/2019GL082867.
(2) Ryerson, T. B.; Camilli, R.; Kessler, J. D.; Kujawinski, E. B.; Reddy, C. M.; Valentine, D. L.; Atlas, E.; Blake, D. R.; de Gouw, J.; Meinardi, S.; et al. Chemical Data Quantify <Em>Deepwater Horizon</Em> Hydrocarbon Flow Rate and Environmental Distribution. Proc. Natl. Acad. Sci. 2012, 109 (50), 20246 LP – 20253. https://doi.org/10.1073/pnas.1110564109.
(3) Ward, C. P.; Sharpless, C. M.; Valentine, D. L.; French-McCay, D. P.; Aeppli, C.; White, H. K.; Rodgers, R. P.; Gosselin, K. M.; Nelson, R. K.; Reddy, C. M. Partial Photochemical Oxidation Was a Dominant Fate of Deepwater Horizon Surface Oil. Environ. Sci. Technol. 2018, 52 (4), 1797–1805. https://doi.org/10.1021/acs.est.7b05948.
(4) Vione, D.; Maurino, V.; Minero, C.; Pelizzetti, E.; Harrison, M. A. J.; Olariu, R.-I.; Arsene, C. Photochemical Reactions in the Tropospheric Aqueous Phase and on Particulate Matter. Chem. Soc. Rev. 2006, 35 (5), 441–453. https://doi.org/10.1039/B510796M.
(5) Barkan, E.; Luz, B. High-Precision Measurements of 17O/ 16O and 18O/ 16O Ratios in CO 2. Rapid Commun. Mass Spectrom. 2012, 26 (23), 2733–2738. https://doi.org/10.1002/rcm.6400.
(6) Ward, C. P.; Armstrong, C. J.; Conmy, R. N.; French-Mccay, D. P.; Reddy, C. M. Photochemical Oxidation of Oil Reduced the Effectiveness of Aerial Dispersants Applied in Response to the Deepwater Horizon Spill. Environ. Sci. Technol. Lett. 2018, 5 (5), 226–231. https://doi.org/10.1021/acs.estlett.8b00084.