Dispatch 21: Oli Oli oxygen!
Chloe Immonen
October 5, 2023
Dispatch 21: Oli Oli oxygen!
Hi, my name is Chloe Immonen, and I work at the Institute of Ocean Sciences (IOS) on Vancouver Island in Canada! Do you like being able to breathe oxygenated air? Well, I have some good news for you! The ocean is full of oxygen! Dissolved oxygen concentrations are primarily set at the sea surface where the ocean interacts with the atmosphere through air-sea gas exchange. The amount of oxygen retained by the ocean depends on the temperature and salinity properties of that specific water body. Colder and fresher water can retain dissolved gases better than warmer and saltier water. In addition to atmospheric mixing, dissolved oxygen is produced by tiny plants called phytoplankton that live in the upper few meters of the water column, where the sunlight can penetrate. They photosynthesize using energy from the sunlight, plus carbon dioxide and water to produce oxygen and sugar. Since the phytoplankton need sunlight to photosynthesize, oxygen concentrations are highest near the sea surface. Oxygen is then consumed during processes like respiration (animals breathing) and decomposition (breakdown of organic matter by bacteria). Those processes most commonly take place deeper in the water column, so it is normal for the oxygen concentration to decrease with depth.
We measure dissolved oxygen concentrations by two different methods. The first is with a sensor mounted on our CTD rosette water sampler. This instrument package is lowered over the side of the ship once we are at a particular station and the CTD logs the profiles of many different water properties (for example, the temperatures, conductivities, dissolved oxygen concentrations, fluorescence, etc.) continuously the whole way down and back up. The second method is through discrete water samples which are gathered by the Niskin Bottles on the rosette.
For us to measure oxygen concentrations, we must be very careful with our sampling and analysis practices. As I’m sure you’re aware, there is a lot of oxygen present in the air around us, so it is easy to contaminate our samples! Since dissolved gas samples can be so volatile, they need to be sampled from our rosette bottles before anything else to reduce contamination risk while interacting with the air once it comes back on board. Our water samplers draw water from the Niskins, careful not to have any air bubbles into the glass flasks. They are promptly “pickled” with two chemicals (manganous chloride and alkaline iodide) to preserve the oxygen concentration present in that specific water sample. The flask is then capped, ensuring no bubbles have been introduced. The sample is then shaken to ensure the pickling chemicals are thoroughly mixed with the sample. After 30 minutes, the samples are shaken a second time before a water seal is added to the top of the flask to ensure no air can escape or enter.
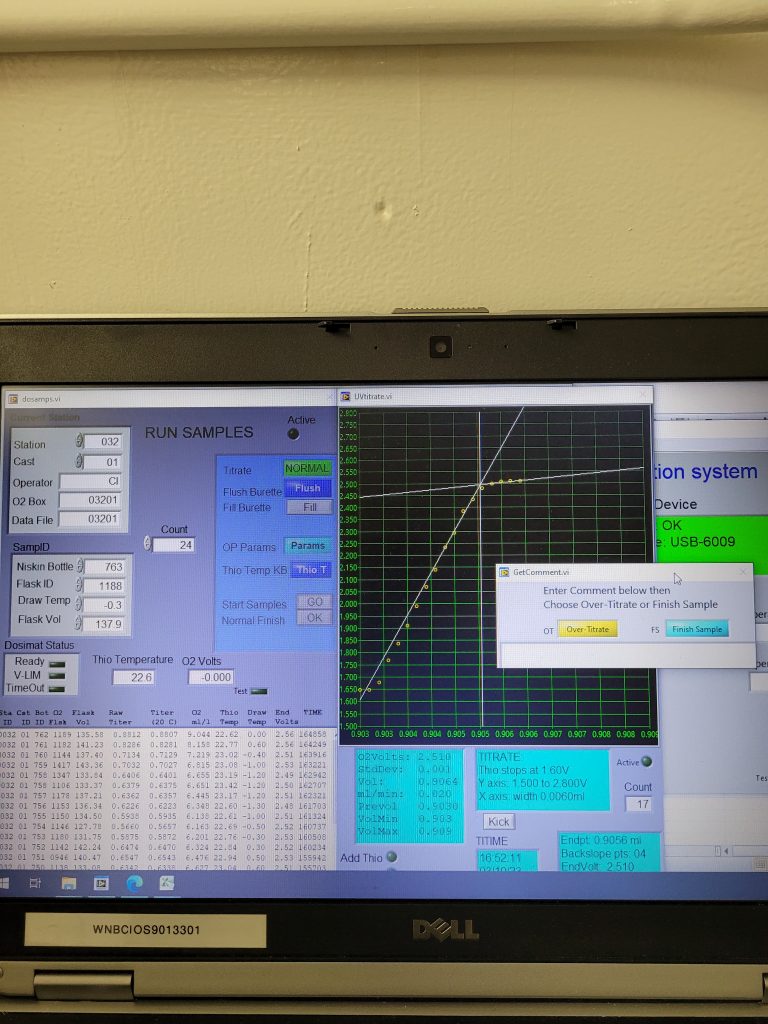
When the time for analysis comes, the analyst removes the water seal and opens the cap. They then add sulfuric acid and a magnetic stir bar to dissolve the precipitate formed by the pickling process. Placing the sample flask on a stir plate in the titration kit (figure 1), the analyst checks to make sure all precipitate is dissolved, and no bubbles are present before placing the titration burette tip in the sample flask. At this stage, the sample is a pretty amber color (provided by the iodine in the pickling chemicals, figure 2). The titration magic can now begin! Yet another chemical (called thiosulphate, or thio for short) is continuously added to the sample while a UV light is shone through until the light detector in the titration kit detects that the sample has become clear in colour (indicated by no more change in voltage with added thio, (figure 3) and the titration is complete. The computer produces a titration curve with voltage on the y-axis and volume thio added on the x-axis (figure 4). The program then calculates the oxygen concentration based on a stoichiometric formula of the chemical reaction taking place.